By sharing electrons or 2. Hold atoms together in compounds.
Solved Er 02 Homework Building Vocabulary Which Chemical Chegg Com
Metallic bonds two or more metals bonded together.

. Chemical Bonds are the force that holds atoms together. Type of bond when two atoms share electrons. There are two main ways that atoms can bond together to form molecules.
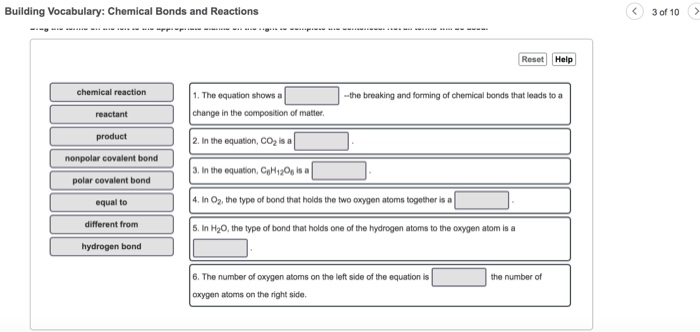
By sharing electrons or 2. In O2 the type of bond that holds the two oxygen atoms together is a 5. Ionic bonds involve the transfer of one or more electrons from one atom to another.
A chemical bond is the force of attraction that hol ds two atoms together as a result. Chemical bond a force of attraction that holds two atoms together Valence electrons the electrons in the outermost energy level of an atom Ionic bond the force of attraction between oppositely charged ions Ions charged particles that form during chemical changes when one or more valence electrons transfer form one atom to another. Two or more atoms bonded together.
Ionic bonds involve the transfer of one or more electrons from one atom to another. By sharing electrons or 2. Up to 24 cash back 5 Chemical energy Potential energy associated with the covalent bonds that hold atoms and molecules together 6 Matter Anything with mass and volume 7 Element A substance that cannot be broken down chemically into another substance 8 Compound A substance made of two or more different elements 9 Emergent property.
Covalent Bond A chemical bond in which two atoms share a pair of valence electrons. There are two main ways that atoms can bond together to form molecules. General chemistry expand collapse global location chemical bonding worksheet last updated nov 5 2017.
One electron donor and one electron acceptor The process by. Made up of metal and nonmetal atoms joined together. There are two main ways that atoms can bond together to form molecules.
A compound consists of molecules of the same type. By transferring electrons and creating two oppositely charged atoms Covalent bonds involve the sharing of one or more electrons between atoms. A covalent bond in which the electrons are shared equally by t.
A dipole-dipole bond in which a hydrogen atom covalently bonde. A chemical bond in which one atom loses an electron to form a positive ion and the other atom gains to electron to form a negative ion. The electrons involved in bonding are usually those in the _____valence shell.
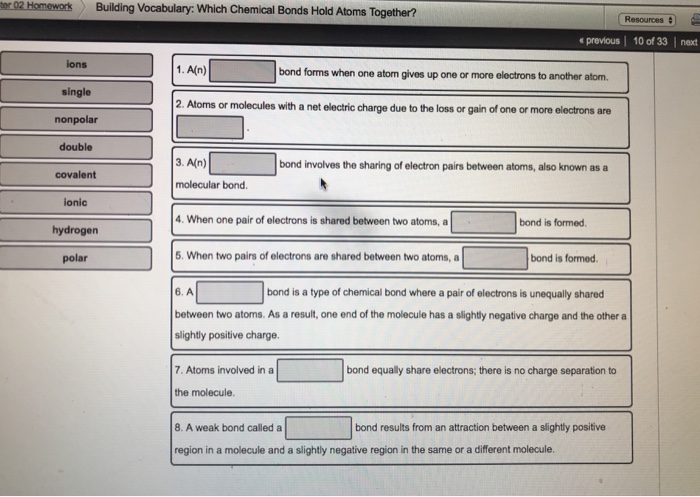
In the equation CH120 isa reactant product nonpolar covalent bond polar covalent bond equal to different from hydrogen bond. When only one pair of electrons is shared between atoms. 2 Atoms or molecules with a net electric charge due to the loss or gain of one or more electrons are _____.
Positively or negatively charged atoms. Holds two atoms together as a result of the rearrangement of electrons between them. Chemical bond- What chemists call the attraction that holds atoms together.
A chemical bond is the force that holds atoms together in a compound. Four types if Bonding. By transferring electrons and creating two oppositely charged atoms Covalent bonds involve the sharing of one or more electrons between atoms.
Polyatomic Ion A covalently bonded group of atoms that has a positive or negative charge and acts as a unit. Chemical bonds and reactions. By transferring electrons and creating two oppositely charged atoms Covalent bonds involve the sharing of one or more electrons between atoms.
A particle that is electrically charged positive or. Chapter 8 Concepts of Chemical Bonding Chemical Bonds. In the equation CO2 is a 3.
A molecule is the smallest part of a compound and consists of two or more different atoms that are bonded together. Chemical Forces Hold Atoms Together in Molecules Three types of chemical forces Ionic Bonds electrons exchanged to form ions Covalent Bonds. Which Chemical Bonds Hold Atoms Together.
A chemical bond is formed when electrons are shared between two atoms. Chemical bonds _____that hold atoms together in compounds. Most elements in compounds want to gain _____ configuration.
Compound made up of two or more molecules. A substance formed by the chemical combination of two or more elements in definite proportions. Atoms held together by covalent bonds The transfer of electrons to each other.
The attractive force that holds atoms or ions together. The Nature of Bonding There are several major types of bonds. Chemical Bonding Chemical Forces Hold Atoms Together in.
The properties of a compound are different from the properties of the elements that it is made of. A chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Chemical Names and Symbols.
Where two pairs of electrons are shared between the atoms rather than just one pair. Small number slightly below a chemical symbol in a chemical formula that tells you how many atoms of a specific element a chemical formula has. 1 An _____ bond forms when one atom gives up one or more electrons to another atom.
Ionic bonds involve the transfer of one or more electrons from one atom to another. Covalent bonds electrons are shared between atoms. Molecular covalent bonding- The valence electrons are shared between parts or groups of atoms.
Chemical bonds are the attractive forces that hold atoms together in the form of compounds. Ionic bonds electrons are transferred between atoms creating cations and anions. Building Vocabulary After you read this section reread the.
Ionic covalent and metallic bonds are three most common types of bonds. Ionic bonding- The valence electrons are transferred from one atom to another. Molecule A neutral group of atoms that are joined together by one or more covalent bonds Polar Covalent Bond A covalent bond in which atoms have been stripped of their electrons.
There are three types of bonds. In H20 the type of bond that holds one of the hydrogen atoms to the oxygen atom is a 6. Formed when one or more electrons are transferred from one atom to another.
Bonding Bridge Building. 3 An _____ bond involves the sharing of electron pairs between atoms also known as. A covalent bond in which electrons are not shared equally.

Solved Concept Hw Locabulary Which Chemical Bonds Hold Chegg Com
Solved Building Vocabulary Chemical Bonds And Reactions 3 Chegg Com
0 Comments